ground state electron configuration of co3|Electron Configuration for Co, Co2+, and Co3+ (Cobalt and : iloilo Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy . Tingnan ang higit pa The courtyard at 888 Brannan Street is conceived to address the changing work habits of San Francisco’s tech workers and illuminates the important role. Home; Projects; Products; . A significant design challenge was how to treat the existing overhang of the Jewelry Center which created a shaded and oppresive space. The design solution .
ground state electron configuration of co3,The ground state electron configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. This electron configuration shows that the last shell of cobalt has two electrons and the d-orbital has a total of seven electrons. Therefore, the valence electrons of cobaltare nine. There are two types of cobalt ions. The . Tingnan ang higit paThe total number of electrons in cobaltis twenty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy . Tingnan ang higit pa Co3+:[Ar]4s3d5. Answer link. The electron configuration of "Co"^ (3+) is ["Ar"] 4s 3d^5. "Co" is in Period 4 of the Periodic Table, and "Ar" is the preceding noble . Wayne Breslyn. 757K subscribers. 988. 141K views 4 years ago. To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We . Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons .
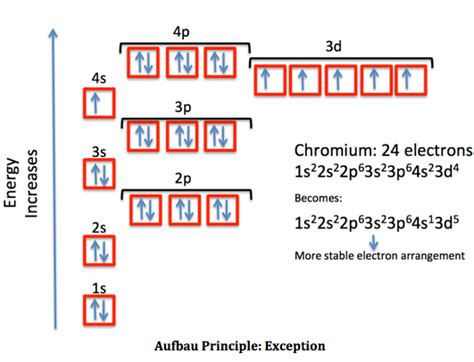
Write the expanded and shortened ground state electron configuration for Cr. Write the expanded and shortened ground state electron configuration for Cu. .
Derive the predicted ground-state electron configurations of atoms. Identify and explain exceptions to predicted electron configurations for atoms and ions. Predict the charge of common metallic and nonmetallic .
Electronic Configuration Of Co3+ is: Co3+:1s2 2s2 2p6 3s2 3p64s13d5. The 4s and 3d sublevels are nearly identical in energy, so the ion can become more stable by moving . Electronic configuration of Co³⁺ - Chemistry Stack Exchange. Ask Question. Asked 4 years, 7 months ago. Modified 4 years, 7 months ago. Viewed 149 .We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams . 329 views. Geometry Of Molecules.
write the correct electron configuration for the Co3 ground state. University of Ottawa; Principles of Chemistry; Question; Subject: . 11 months ago. write the correct electron configuration for the Co3+ ground state. Like. 0. All replies. Answer. 11 months ago. Cobalt (Co) is a d-block element and a transition metal with an atomic number .
ground state electron configuration of co3For example, calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. When a Ca atom loses both of its valence electrons, the .6. (15 points) If we assume that Co (III) oxide and phosphate form an octahedral complex with a strong ligand field, write the ground state electron configuration of Co3+ ion, use the energy level diagram in CHEM 112 datasheet to draw its expected d-orbital splitting diagram, and predict whether the complex is diamagnetic or paramagnetic. The electron configuration for Co is [Ar] 3d7 4s2. When removing 3 electrons to make Co into Co3+, the first electrons that leave are from the outer shell 4s2, even though we filled it first as it is higher in energy. So, 2 electrons from 4s2 are removed. One more has to be removed from the 3d orbital. This leaves 3d6, resulting in 6 valence .
Electron Configuration for Co, Co2+, and Co3+ (Cobalt and Cr and Cu have unique configurations because they steal electrons from the 4s because they prefer to be half-full or full orbitals. So if you have 4 electrons in the 3d, like in Cr, it is more energetically favorable to have 5 electrons in the 3d and have a half-filled subshell since the 3d has 5 orbitals, so you would pull an electron from the 4s.
Question: Which 3+ ion has the ground state electron configuration [Ar] 3d3 ? Co3+ Fe3+ Mn3+ V3+ Cr3+ Which 3+ ion has the ground state electron configuration [Ar] 3d 3? Co 3+ Fe 3+ Mn 3+ V 3+ Cr 3+ There are 3 steps to solve this one. Who are the experts? Experts have been vetted by Chegg as specialists in this subject.

View Solution. Q 5. Which one of the following ions has an electronic configuration of [Ar]3d6 ? (Atomic N umber: M n = 25,F e =26,Co = 27,N i = 28) View Solution. Click here:point_up_2:to get an answer to your question :writing_hand:what is the electron configuration of co3.ground state electron configuration of co3 Electron Configuration for Co, Co2+, and Co3+ (Cobalt anda) Write ground-state electron configuration in complete form (instead of the condensed form) for the Zn^ (2+) ion. Express the answer as a series of orbitals. b) State whether or not the ion will be paramagnetic due to the presence of unpaired electrons. Write the electron configuration for the Ca2+ ion. question. Co3+ is Cobalt with three electrons removed (It's positive by 3). So, the abbreviated notation for Cobalt, minus three electrons (Coming from 4s and 3d) is: [Ar]4s1, 3d5.PROBLEM 3.1.12 3.1. 12. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. Write the electron structure of the two cations. Answer. 1.9A: Ground State Electronic Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Olivia Allsman. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing orbitals and . Rule 2 predicts a F F state since that is the highest multiplicity with L = 3 L = 3: So the ground state is from this more narrowed list: 3F4 3 F 4, 3F3 3 F 3, 3F2 3 F 2. Rule 3 predicts the lowest J J term since the d shell is less than half full. That is the J = 2 J = 2 state. Therefore for this system, the atom will have a ground-state .Step 1. The electronic configuration is usually represented by a series of numbers and letters. Each energy level is designated by a principal quantum number ( n), and each sublevel within an energy level is represented by a letter ( s, p, d, f). The combination of the principal quantum number and the sublevel designation provides information .This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Predict the ground‑state electron configuration of each ion. Use the abbreviated noble gas notation. Cr2+Cr2+: Cu2+Cu2+: Co3+Co3+: Predict the ground‑state electron configuration of each ion.Which of the following is the ground-state electron configuration of a C^{3-} ion? a. 1s^22s^22p^4. b. (He)2s^22p^6. c. (He)2s^1. d. 1s^22s^22p^5. e. 1s^22s^22p^63s^1. Using noble gas notation, write the electron configuration for the copper(II) ion. Write out the electron configurations for each of the following ions. a. K+ b. Mg2+ c. Al3+Question: Write the full electron configurations of cobalt metal, Co, and one of its ions, Co3+. electron configuration of Co: 1s22s22p%3s23p63d7452 electron .
Its atomic number will show how many electrons are in its ground state electron configuration. Based on the periodic table trends and the rules/principles outlined in the lesson, write the lowest .

Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
ground state electron configuration of co3|Electron Configuration for Co, Co2+, and Co3+ (Cobalt and
PH0 · What is the electron configuration of "Co"^"3+"?
PH1 · Electronic configuration of Co³⁺
PH2 · Electronic Configuration Of Co3+ Chemistry Q&A
PH3 · Electron Configuration for Cobalt (Co and Co2+, Co3+)
PH4 · Electron Configuration for Cobalt (Co and Co2+, Co3+)
PH5 · Electron Configuration for Co, Co2+, and Co3+ (Cobalt and
PH6 · Electron Configuration for Co, Co2+, and Co3+ (Cobalt and
PH7 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH8 · 3.1: Electron Configurations
PH9 · 1.9A: Ground State Electronic Configurations
PH10 · 1.7: How to Write a Ground State Electron Configuration